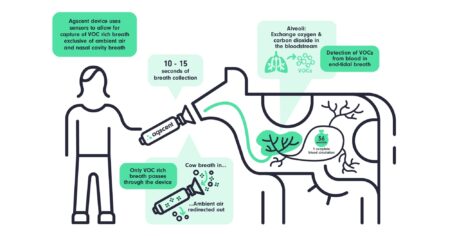
Plant-based products’ use of the term “milk” has caused friction for decades among consumers, dairy producers, and the companies that produce milk alternatives.
Now, the American Butter Institute is urging the U.S. Food and Drug Administration to take action against Country Crock’s Dairy Free Salted Butter, asserting the product is misbranded and violates multiple federal labeling laws. In a letter dated June 25, 2025, ABI Executive Director Christopher Galen emphasized that the product, labeled as “butter,” fails to meet the legal definition and creates a misleading impression for consumers.
ABI’s central argument rests on the long-standing statutory definition of butter, which dates back to the Act of March 4, 1923, now codified in 21 U.S.C. § 321a. According to this statute, butter is:
“… made exclusively from milk or cream, or both, with or without common salt, and with or without additional coloring matter, and containing not less than 80 percent by weight of milk fat …”
Country Crock’s “dairy free” product contains no milk or cream. Instead, it’s made from plant-based oils, pea protein, and natural flavors. Despite this, the ABI argues that the product is labeled prominently with “butter” in the title and features imagery traditionally associated with dairy — like barns and butter sticks.
The “dairy free” wording also appears to be intentionally made smaller when compared with almost all of the other text on the packaging.

ABI argues that this packaging misleads consumers into believing they are purchasing a dairy product when, in fact, the spread contains no dairy and doesn’t qualify as butter under federal law. The group calls this an “egregious attempt to mislead” and urges the FDA to take enforcement action.
Federal law offers ABI a strong foundation. The Food, Drug, and Cosmetic Act explicitly prohibits misbranding of food products:
- Section 403(a)(1) prohibits labeling that is false or misleading in any particular.
- Section 403(b) prohibits offering a product for sale under the name of another food.
- Section 403(c) requires a product that imitates another food to be labeled as “imitation”, followed by the name of the food it imitates.
In addition, 21 CFR 101.3 and 102.5 outline the FDA’s implementation of these rules:
- A product must use its common or usual name, and cannot be confusingly similar to another food’s name if it’s not actually that food.
- If a product resembles and substitutes for another food but is nutritionally inferior, it must be labeled as an imitation. ABI points out that Country Crock’s dairy-free spread lacks key nutrients found in real butter, like calcium, which further triggers the requirement for the “imitation” label.
Country Crock has not publicly responded to ABI’s complaint.
What this means for labeling
Given both the statutory and regulatory frameworks, ABI’s complaint seems to have merit. The FDA has long required that standardized food names — like “butter” — be used only when products meet the exact legal definition. Labeling a plant-based, dairy-free product as “butter” does not comply with that definition and risks misleading consumers.
Even with a clarifying phrase like “dairy free,” the product still capitalizes on the trust and familiarity consumers have with butter. Unless prominently labeled as an “imitation” or “plant-based spread,” the use of “butter” on such packaging appears to violate both the spirit and letter of federal food labeling law.
The ABI’s letter is not a formal enforcement action, but a public and regulatory pressure move. The FDA now faces the decision of whether to investigate and possibly take action against Country Crock’s marketing practices.
For now, the issue raises broader questions about how plant-based products are labeled and marketed — especially when they mimic traditional, animal-based foods. With growing consumer interest in dairy alternatives, the debate over what qualifies as butter, milk, or cheese is likely far from over.
»Related: FDA: Guidance on plant-based beverages’ use of ‘milk’


:max_bytes(150000):strip_icc()/SMinnesota1025-2000-83ff162cd24b4b0fb5065466fe80a0d5.jpg)
:max_bytes(150000):strip_icc()/r4d092124_rrd1-5d0657ac040743a2a4c5ebf998902de0.jpg)





:max_bytes(150000):strip_icc()/Markets-1-Cattle-dramatic-up-5-0687ebaec7fc487bac9c10a7f442eea8.jpeg)