Current news headlines have highlighted a 16 percent rise in U.S. sales of medically important antibiotics that are used in food-producing animals in 2024. The news stories are based on information from the U.S. Food and Drug Administration’s data officially released last month. However, the FDA’s report has a broader context than the news stories imply.
One of those articles published this week, by The Guardian, was quick to point to rising fears of “superbugs,” drug-related cancer risks, and other concerns tied to antibiotic use in livestock (this is the same outlet that targets any agriculture that’s not “organic”).
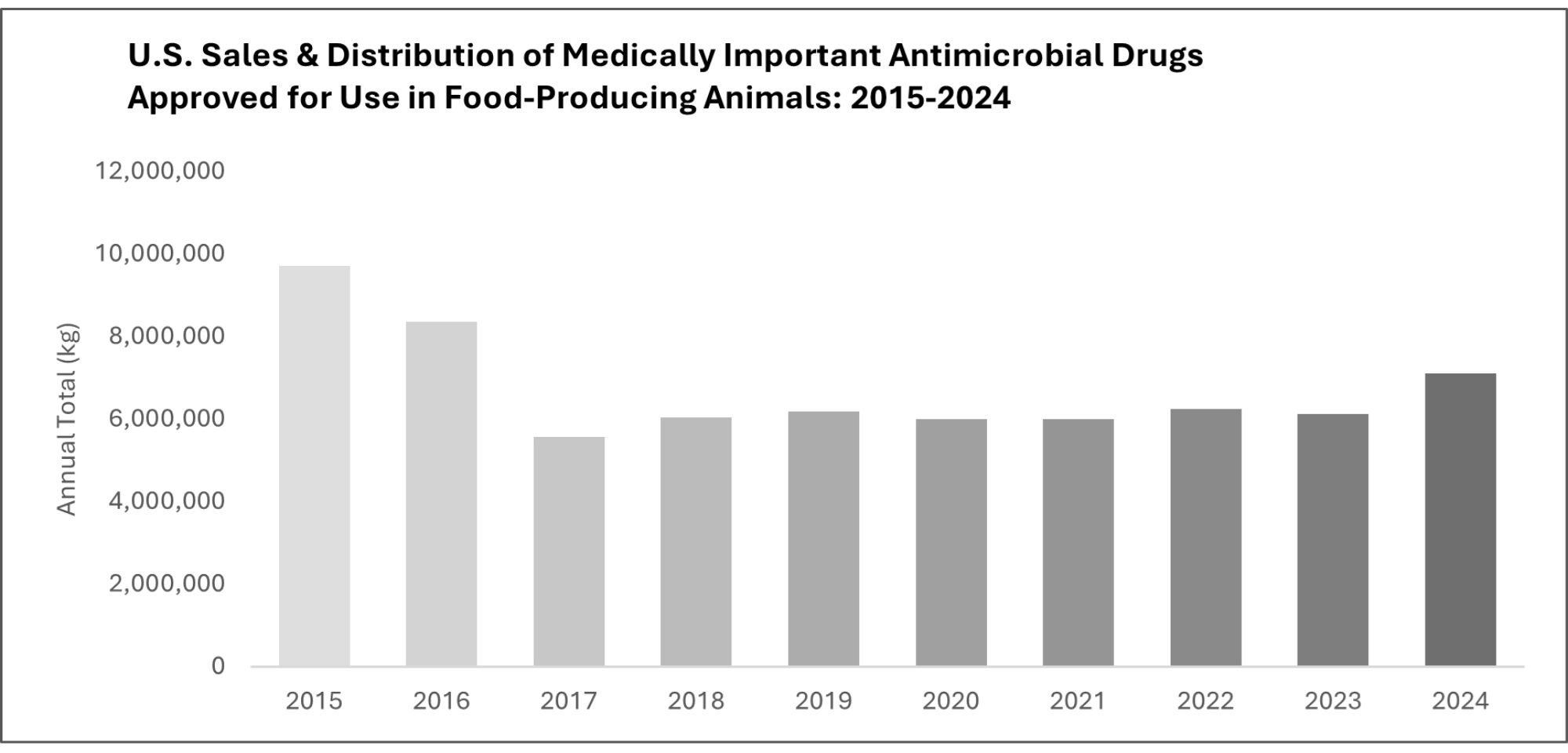
So what are the facts here? According to the FDA’s Center for Veterinary Medicine, sales and distribution of medically important antimicrobial drugs rose 16 percent between 2023 and 2024. That increase marks the largest single-year rise since the agency began tracking the data a decade ago.
Medically important antimicrobial drugs are administered by veterinarians and used specifically to treat illness. According to research published in the Journal of Antimicrobial Chemotherapy in 2024, the use of antibiotics in food animals selects for bacteria resistant to antibiotics used in humans, and these might spread via the food system to humans. However, although some antibiotics are used both in animals and humans, the journal also notes that most of the resistance problem in humans has arisen from human use.
FDA statistics indicate that antibiotic sales in 2024 continue to be 27 percent lower compared to the peak levels prior to the implementation of significant regulatory changes (like the Veterinary Feed Directive) that altered the ways in which antibiotics were used within livestock production in the mid 2010s. In the years following 2017, antibiotic sales were stable or decreased due to increased veterinary regulation. In 2022 and 2023, over-the-counter animal antibiotics were removed from the market.
In other words, the 2024 increase represents a rebound from recent lows, not a return to pre-reform levels.
Sales data are not the same as on-farm use
A key limitation of the FDA report is frequently overlooked: Sales and distribution data do not measure how much antibiotic is actually administered to animals.
“Sales volume may fluctuate over time in response to various factors, including changing animal health needs, changes in animal populations, and changes in animal production practices,” the news release said. “It is important to note that antimicrobial sales data do not necessarily reflect how much of the drugs are ultimately used in animals, only the volume that is sold.”
Products may be purchased in anticipation of need, held in inventory, or used over multiple production cycles.
At present, there is no national system in the United States that tracks real-time antimicrobial use across livestock operations. As a result, year-to-year changes in sales can reflect a range of factors, including animal health challenges, shifts in livestock populations, or changes in production practices — not necessarily increased routine use.
That said, veterinary and industry reports from 2024 indicate that there was increased disease pressure in some livestock segments.
For instance, the biomass-adjusted sales data, which account for changes in the number of animals and the weights of animals, indicate an increased intensity of usage for some of the species even as the overall animal population was declining. This implies that the disease management challenges, rather than the structural changes in the antibiotic policy, could be the cause of the increased usage.
The 2024 jump wasn’t spread evenly across drug classes. Most of the increase came from a handful of medically important categories: tetracyclines rose about 20 percent and remained the largest class by volume, aminoglycosides increased roughly 37 percent, and lincosamides climbed about 11 percent.
Tetracyclines accounted for 69 percent of all antibiotics sold, followed by macrolides at 8 percent, penicillins and aminoglycosides at 7 percent each, sulfonamides at 5 percent, lincosamides at 3 percent, cephalosporins at 1 percent, and fluoroquinolones at less than 1 percent. Penicillins, however, dropped 14 percent.
Swine accounted for 43 percent of antibiotic sales, followed by cattle at 41 percent, turkeys at 11 percent, chickens at 4 percent, and other or unspecified species at 2 percent.


The FDA’s report includes an interactive dashboard with biomass-adjusted 2024 sales data, which puts annual antibiotic sales in context by adjusting for each species’ estimated biomass calculated as population multiplied by average weight.
Because livestock populations and weights shift year to year, the biomass adjustment helps normalize the data for more meaningful comparisons.
Using that approach, biomass-adjusted sales — a proxy for the intensity of antimicrobial sales relative to animal population — increased for several drug classes in 2024 after declining in some categories in 2023.
Although poultry antibiotic sales increased significantly, the rise came after years of declining use and during a period marked by increased disease pressure, including widespread outbreaks of highly pathogenic avian influenza.
Cattle sales, meanwhile held relatively steady even as cattle inventories have decreased over the previous three years.
Tetracyclines are widely used in both beef and dairy for bovine respiratory disease, anaplasmosis, and some reproductive tract infections, in part because they’re broad-spectrum and available in multiple formulations.
In swine, aminoglycosides and sulfonamides are commonly used for enteric and respiratory issues. Fluoroquinolones and tetracyclines play an important role in treating more complex respiratory disease and systemic infections, while lincosamides are often used more specifically for conditions such as swine dysentery, mycoplasmal pneumonia, and infectious arthritis.


Regulatory oversight remains in place
Medically important antibiotics used in food animals continue to be regulated by the FDA and must be used under the oversight of a veterinarian. Growth promotion uses of antibiotics continue to be prohibited, and the drugs must be used in accordance with the approved label directions.
Despite the difficulties in enforcing the law and the debates about the use of antibiotics in agriculture, the regulatory system for the use of antibiotics in animals today is much more restrictive than it was ten years ago.