Biological crop protection products are sometimes seen as a potential step for production systems, but experts say the biggest hurdle has been reliability. Growers facing tight spray windows, high disease pressure, and rising input costs need tools that perform consistently across regions, seasons, and application methods.
That context is central to Biotalys’ latest milestone: the agtech company announced that the U.S. Environmental Protection Agency has issued final approval for its first biofungicide, EVOCA, a protein-based, precision biocontrol product designed to manage destructive fungal diseases in grapes, strawberries, and hemp.
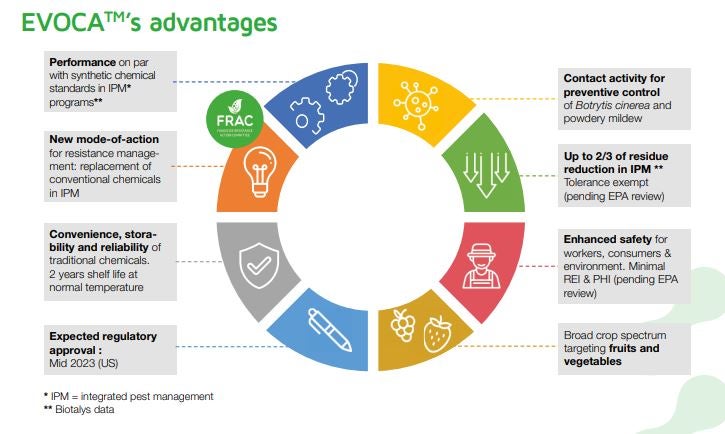
EVOCA is the first product developed using Biotalys’ proprietary AGROBODY technology platform, which relies on antibody-derived proteins similar in concept to those used in human medicine to precisely target and neutralize fungal pathogens.
Biotalys engineers small, stable proteins intended to control diseases like botrytis (gray mold) and powdery mildew while minimizing risk to beneficial organisms and the environment. Biotalys says EVOCA has been validated through more than 600 field trials across climates, crops, soil types, production systems, and levels of pathogen pressure. The product’s mode of action has been recognized by the Fungicide Resistance Action Committee as code F10 — an important designation as resistance pressure continues to build in many fungicide programs.
“This approval marks a major regulatory milestone for EVOCA and moves us closer to delivering a new, sustainable tool for farmers to protect their crops,” said Kevin Helash, CEO of Biotalys. “The product has an entirely new mode of action to target fungal diseases, highlighting the uniqueness of Biotalys’ technology platform as a pathway to discovering many new modes of action in the coming years. The EPA’s decision reinforces the potential of our technology to help shape the future of agriculture and is a testament to the dedication of our entire team.”
With federal registration in place, Biotalys said it can proceed with state-level dossiers, including California and Florida, two of the most significant fruit and vegetable production regions in the U.S.
The company also noted that EVOCA is designed for flexibility, including use in both pre- and post-harvest applications, compatibility with a range of application methods, and integration into existing farm management practices. Separately, Biotalys said the EPA posted a final rule in late October 2025 exempting EVOCA’s active ingredient residues on treated crops from tolerance requirements, meaning no maximum residue limits will apply.
Looking ahead, Biotalys plans to advance its next-generation formulation, EVOCA NG, which contains the same active ingredient as EVOCA but is being developed with enhanced formulation and production methods. The company expects the regulatory review for EVOCA NG to be shorter and has outlined a longer-term commercialization path that also includes expansion efforts in Europe and additional markets.


